Introduction
Standard androgen-suppressing medications used for ADT (as well as surgical castration) deprive the body of not just testosterone (T), but also estradiol (E2). This happens because E2 is made directly from T in men. It is the loss of E2 that leads to the symptoms of menopause that are distressful for women. It is also the loss of E2 —not the loss of T—that accounts for many of the bothersome adverse events (AEs) of ADT experienced by PCa patients.
The AEs of ADT include hot flashes, poor sleep, daytime fatigue, increased blood sugar, increased bad cholesterol, and diminished mental acuity of the sort that cancer patients call “brain fog.” The loss of E2 also causes a decrease of bone mineral density (osteoporosis) and, consequently, an increased risk of fractures. Collectively, the AEs arising from lack of E2 significantly diminish PCa patients’ quality of life.
If one’s PCa is not a rare form that is estrogen-sensitive (which can be assessed by doing germline genetic screening; e.g., for BRCA2 mutation), then there is no oncological benefit for PCa patients being deprived of their E2.
Hot flashes happen in men (when doing ADT) and women (at menopause) when there is an abrupt drop in estradiol levels in the body. Neighboring neurons in a region of the brain called the hypothalamus regulate both reproductive hormone levels and the body’s temperature. A sharp drop in estradiol levels cause some neurons in that region, which are devoted to reproductive hormones levels, to fire. Because they are next to the thermoregulatory center in the brain there appears to be crosstalk between the two centres.
Definitions:
- High dose tE2 is a dose high enough to suppress T to < 20 ng/dL.
- Low dose tE2 is a dose high enough to reduce the most bothersome menopausal side effects of ADT (i.e., hot flashes and osteoporosis) to an acceptable level, without inducing excessively bothersome gynecomastia (enlarged breasts) and mastalgia (breast/nipple soreness).
Treating Hot Flashes in Post-Menopausal Women with tE2:
We assume that men respond to tE2 therapy in a similar fashion as do post-menopausal women, especially regarding reducing hot flashes and treating osteoporosis.
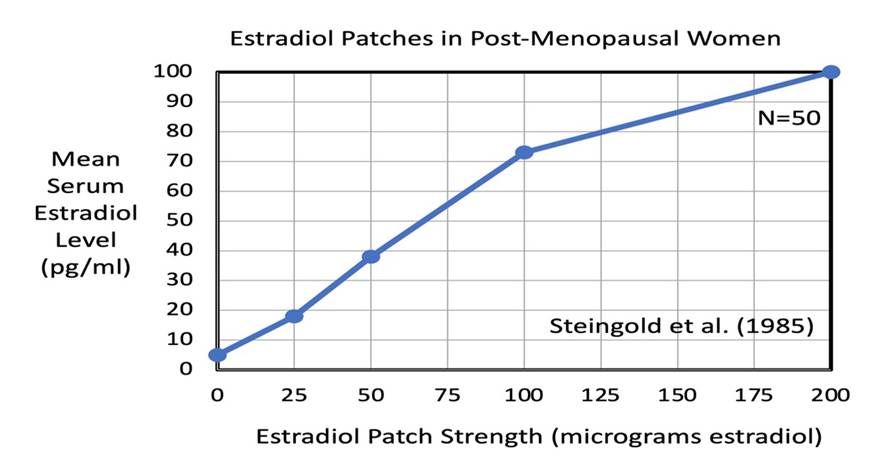
Steingold et al. (1985) studied the use of tE2 patches to treat hot flashes for 50 post-menopausal women. Four sub-groups of these 50 women used tE2 patches with either 25, 50, 100, or 200 micrograms of E2 (delivered over 24 hours). Their patches were changed twice a week and used for a total of 20 days. The frequency of hot flashes (#/hr) was measured for each woman.
A linear, dose-dependent relationship between increasing E2 patch strength and increasing serum E2 concentration was reported by Steingold et al. As illustrated below, the four different patch strengths of 25, 50, 100, and 200 (mg E2/24hrs) of E2 produced mean serum E2 concentrations of 18, 38, 73, and 100 pg/ml, respectively.
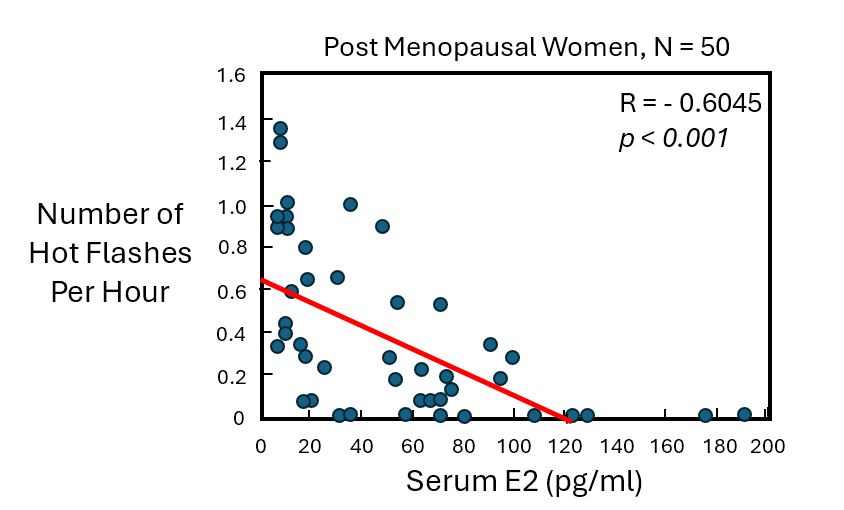
The next graph from Steingold et al. shows the hot flash frequency (#/hr) for post-menopausal women as a function of serum E2 concentration. As expected, the frequency of hot flashes decreased as the serum E2 level increased. On average, a 50% reduction in hot flash frequency (#/hr) was observed at serum E2 concentration of ~ 60 pg/ml. At serum E2 > 120 pg/ml, hot flashes were completely eliminated (in this small study). The red line is a best-fit regression line to the blue data points, with a negative correlation coefficient R = -0.6045 (p < 0.001).
Given the large scatter in hot flash frequency observed for these post-menopausal women, it is likely that men on ADT will individually need to adjust their tE2 dosing over a period of weeks-to-months to achieve a dose that is most effective for them.
Treating Hot Flashes in Men on ADT with tE2
Russell et al. (2022) performed a 6-month, randomized, placebo-controlled trial of n = 78 men (average age = 72 years), all undergoing ADT with standard ADT drugs. One-half of the men (n = 39) applied a daily dose of 0.9 mg of low concentration (0.1%) rE2 gel, while the other half of the men (n = 39) applied a daily placebo gel. All of the men kept a diary of hot flash frequency and subjective severity score. Average serum E2 levels were 30 pg/ml (IQR = 6 to 44 pg/ml) for the placebo group, and 95 pg/ml (IQR = 73 to 116 pg/ml) for the tE2 gel group. Note: “IQR” means Inter Quartile Range.
Also, note that the average E2 serum level of 95 pg/ml in the tE2 group is about 2-4 times greater than the normal reference range for a healthy male (20-50 pg/ml). However, based on data from the PATCH/STAMPEDE trial, the dose is well within a safe range for serum E2 concentration without elevating the risk of blood clots.
The graph below shows the median hot flash frequency (#/day) as a function of time (months) over a 6-month period for n = 39 men using the daily tE2 gel.
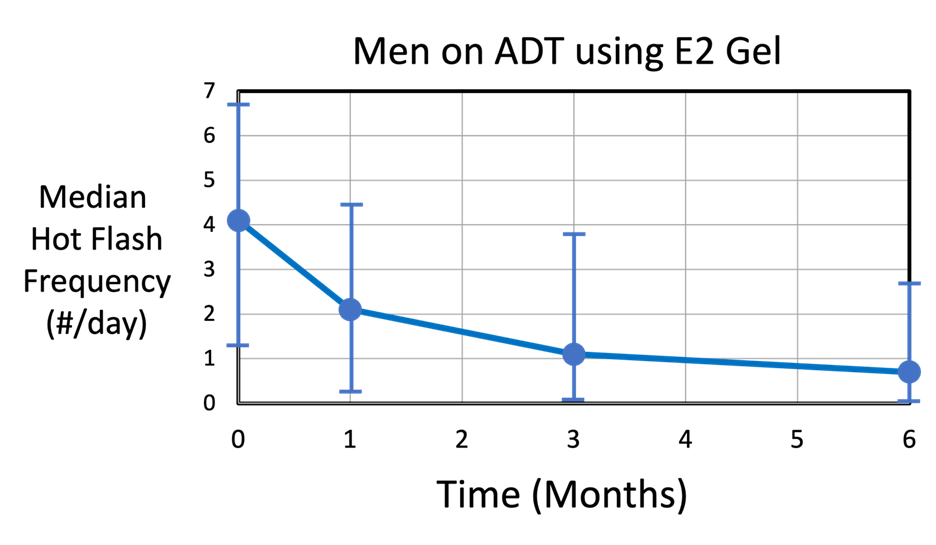
The median hot flash frequency (#/day) decreased continuously from 4 hot flashes per day (IQR = 1.4 to 6.7) down to 0.7 hot flashes per day (IQR = 0 to 2.7) at the end of the 6-month period (p = 0.04). Note that some men had practically no hot flashes after 1 month of tE2 gel use. By comparison, the placebo group (n = 39, not shown) experienced an average of about 2.3 hot flashes/day (IQR = 0 to 5.6), which stayed relatively constant over the 6-month period.
The next graph below shows the median hot flash severity score (no units) as a function of time (months) over a 6-month period for n = 39 men using tE2 gel applied daily.
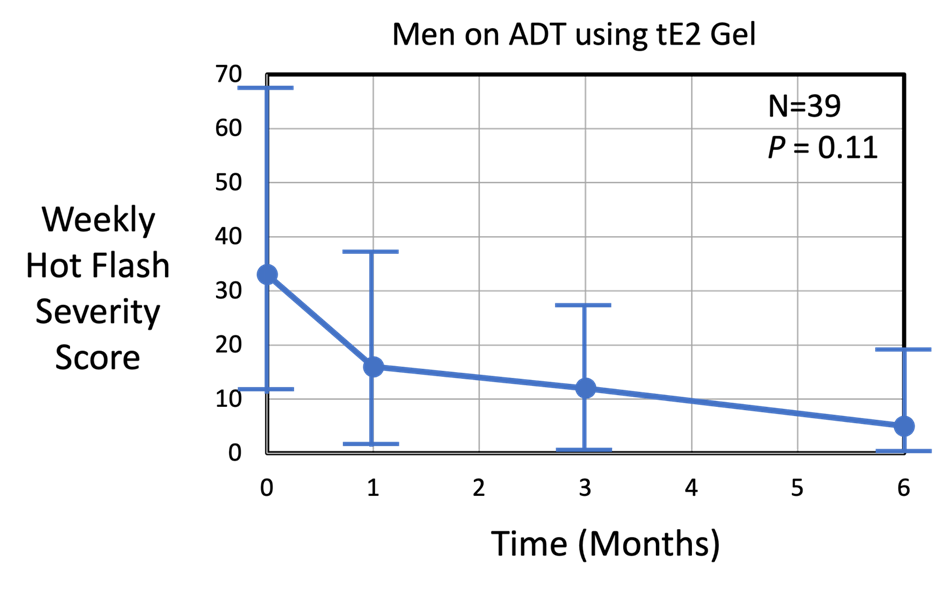
The median weekly hot flash severity score decreased significantly from 33 (IQR = 11.2 to 68.8) to 5 (IQR = 0 to 19) at the end of the 6-month trial (p = 0.11). Note that some men had a severity score of 0-1 after 1 month of tE2 gel use. By comparison, the placebo group (n = 39) (not shown) experienced a median severity score of about 21 (IQR = 0 to 79.5), which stayed relatively constant over the 6-month period.
In summary, Russell et al. showed that men on ADT can significantly reduce hot flash frequency and severity by applying low concentration tE2 gel daily. This can be achieved with an average E2 serum concentration of 95 pg/ml (IQR = 73 to 116 pg/ml). Hot flash frequency and severity continues to decrease over 6 months. Russell et al.’s study confirms the effectiveness of tE2 daily topical gel in treating hot flashes from standard ADT drugs at E2 doses well below those used to suppress testosterone.
From a practical clinical perspective, there is not a specific serum E2 concentration that patients on standard ADT agents should aim to achieve for hot flash control. They may initially want to use a low dose of tE2 and then gradually increase the dose to arrive at a level of hot flash control that they feel is manageable.
Treating Hot Flashes in Men on ADT with High-Dose tE2
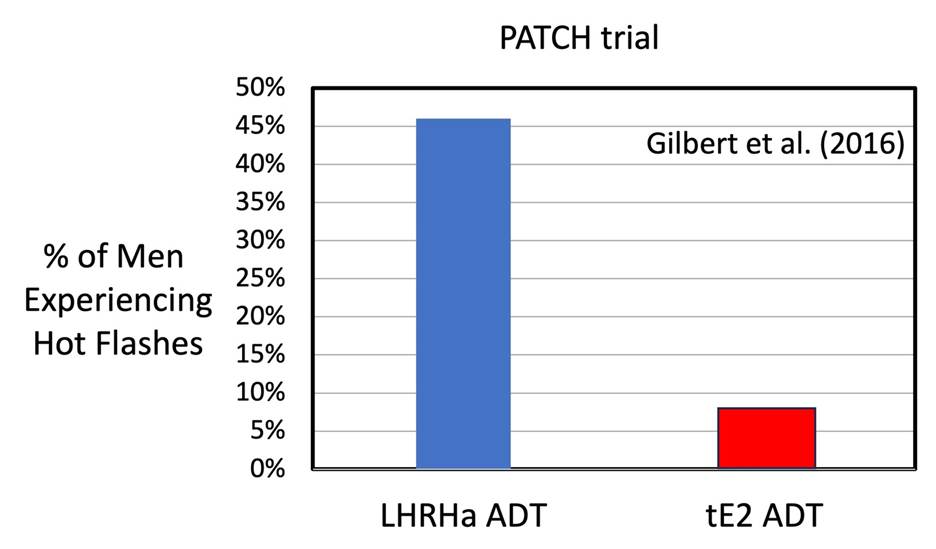
The Patch team in the UK (Gilbert et al., 2016) compared the frequency of Hot Flashes (hot “flushes”) for men on standard LHRHa ADT versus high dose tE2 ADT. They found that 46 % of men on LHRHa ADT experienced hot flashes, while only 8% of men using tE2 ADT experienced hot flashes…a significant reduction.
References:
Steingold KA, Laufer L, Chetkowski RJ, DeFazio JD, Matt DW, Meldrum DR, Judd HL. Treatment of hot flashes with transdermal estradiol administration. J Clin Endocrinol Metab. 1985 Oct;61(4):627-32. doi: 10.1210/jcem-61-4-627. PMID: 3928674.
Duncan C. Gilbert, et al. Quality-of-life outcomes from the Prostate Adenocarcinoma TransCutaneous Hormones (PATCH) trial evaluating luteinising hormone-releasing hormone agonists versus transdermal oestradiol for androgen suppression in advanced prostate cancer, BJUI, (2016) Vol. 119, Issue 5, https://doi.org/10.1111/bju.13687.
Russell N, Cheung A, Grossmann M. Estradiol for the mitigation of adverse effects of androgen deprivation therapy. Endocr Relat Cancer. 2017 Aug;24(8):R297-R313. doi: 10.1530/ERC-17-0153. Epub 2017 Jun 30. PMID: 28667081.
Russell N, Hoermann R, Cheung AS, Zajac JD, Grossmann M. Effects of oestradiol treatment on hot flushes in men undergoing androgen deprivation therapy for prostate cancer: a randomised placebo-controlled trial. Eur J Endocrinol. 2022 Nov 1;187(5):617-627. doi: 10.1530/EJE-22-0318. PMID: 36806623.
