Introduction
Standard androgen-suppressing medications, such as Lupron, Firmagon, etc. (as well as surgical castration) deprive the body of not just testosterone (T), but also estradiol (E2), because E2 in men is made directly from their testosterone. It is this loss of E2 that leads to a loss of bone mineral density (BMD) in both post-menopausal women and men on ADT. The resulting osteoporosis causes weaker bones that significantly increases the risk of fractures, especially in older men and women (see C. Jones et al. (2025)).
Definitions:
High dose tE2 is a dose high enough to suppress T to < 20 ng/dL.
Low dose tE2 is a dose high enough to reduce the most bothersome menopausal side effects of ADT (i.e., hot flashes and osteoporosis) to an acceptable level, without inducing excessively bothersome gynecomastia and mastalgia.
Treating Osteoporosis (in Women) with E2 Patches
A 2-year, placebo-controlled clinical trial was conducted by Sandoz (Novartis, 2023, a manufacturer of the Climara ™ brand of estradiol patches, of n = 175 healthy, hysterectomized, post-menopausal, non-osteoporotic women in the USA. A total of n = 129 women were divided into four sub-groups to receive active treatment with 4 different doses of estradiol patches (25, 50, 75, and 100 mg of E2 delivered over 24 hrs), with n = 46 women receiving placebo patches.
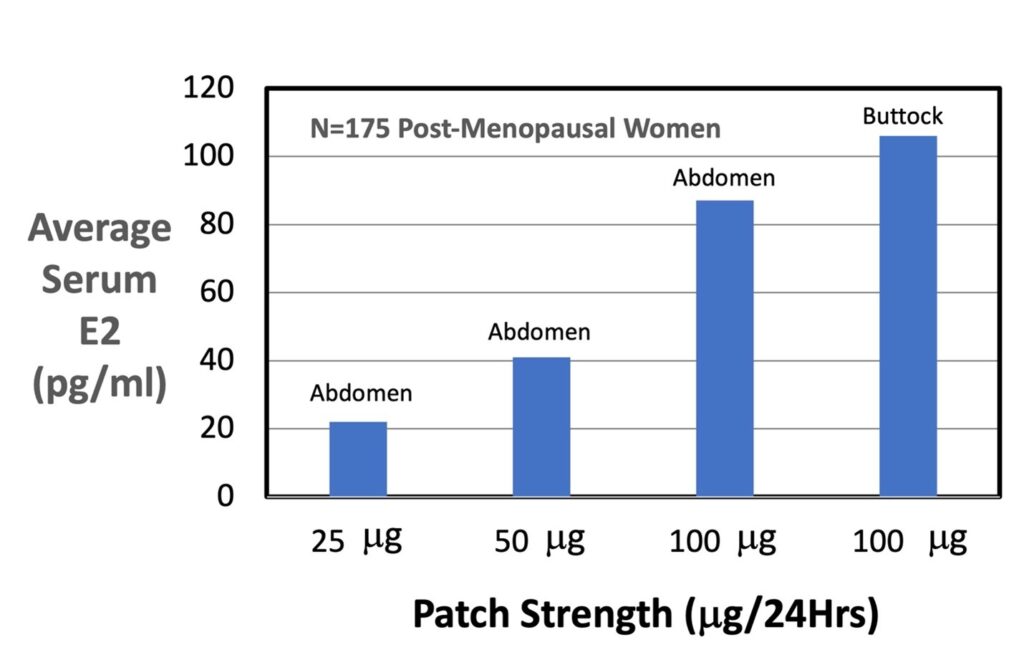
The following table summarizes the mean serum E2 concentrations measured for the different sizes and locations of the Climara™ brand patches used in this study.
| Patch Strength | Patch Size (cm2) | Location | Maximum Serum E2 (pg/ml) | Minimum Serum E2 (pg/ml) | Average Serum E2 (pg/ml) |
| 25 mg | 6.5 | Abdomen | 32 | 17 | 22 |
| 50 mg | 12.5 | Abdomen | 71 | 29 | 41 |
| 100 mg | 25 | Abdomen | 147 | 60 | 87 |
| 100 mg | 25 | Buttock | 174 | 71 | 106 |
The bar graph below illustrates the mean average serum E2 concentration (pg/ml) as a function of patch strength (mg/24Hrs) applied to two different locations: abdomen and buttock. The graph shows a roughly linear positive dose-dependence of average serum E2 concentration (pg/ml) on patch strength (mg/24Hrs), as expected. Interestingly, the mean average serum E2 concentration for patches applied to the buttock location is about 20% greater than patches applied to the abdomen.
Note: we assume than men on ADT will respond to E2 patches in a similar way as post-menopausal women.
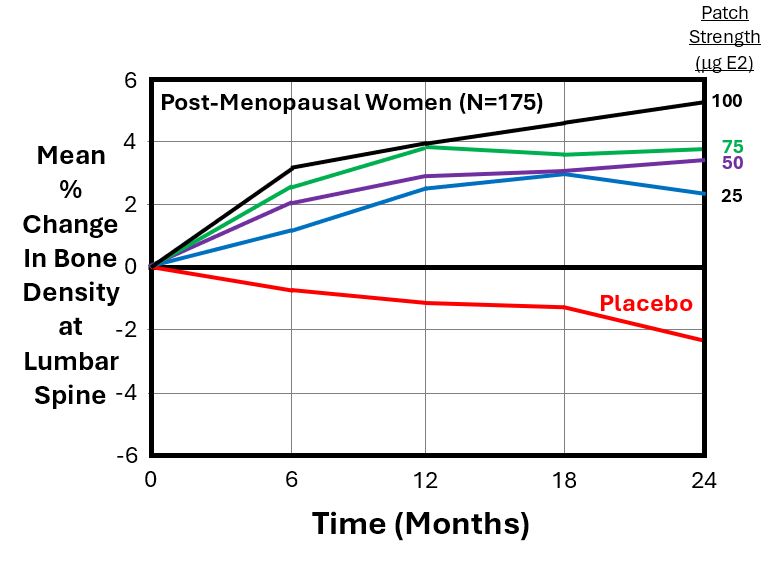
The next graph below from the Sandoz study illustrates the mean % change in bone mineral density (BMD) at the lumbar spine (as measured by DEXA scans) for n = 175 women, as a function of time (months) for the 4 different patch strengths and the placebo patches. All patches were applied to their abdomen.
As expected, the graph shows a rising (dose-dependent) curve, with the greatest increase in BMD (+5.2%) occurring at 2 years for the largest size of patch (i.e., Area = 25 cm2, which delivers 100 mg of E2/24 Hrs). The red line for the placebo patch shows that women who used a placebo patch lost an average of -2.3% of their baseline BMD after 2 years.
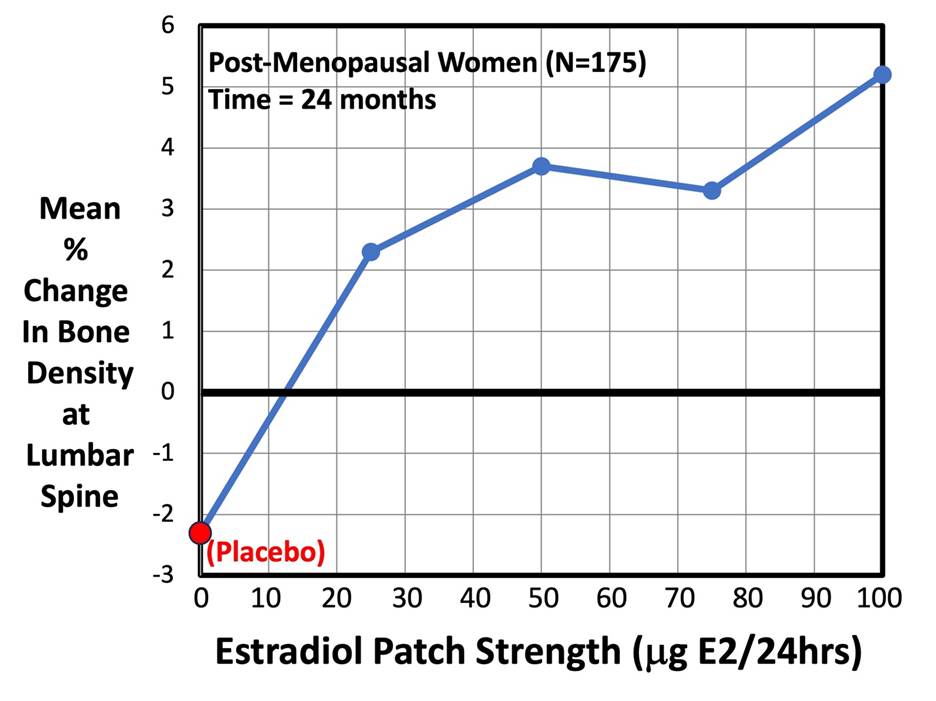
The next graph below, from the same Sandoz study, plots the mean % change in BMD at the lumber spine for n = 175 post-menopausal women at a fixed time = 24 months (2 years).As expected, the graph shows a rising (dose-dependent) curve, indicating that increasing the patch strength causes a corresponding increase in BMD. The greatest increase in BMD (+5.2%) occurred with the largest size of patch (i.e., Area = 25 cm2, which delivers 100 mg of E2/24 Hrs). The red dot at the bottom left-hand corner of the graph indicates women who used a placebo patch. They lost an average of -2.3% of their baseline BMD after 2 years.
Treating Osteoporosis in Men on ADT with High-Dose tE2:
We define “High-Dose tE2” as using a sufficiently high dose of tE2 to cause a serum E2 concentration > 150 pg/ml.
The negative effect of E2 deficiency on bone health in post-menopausal women is well established, but only in the last 20–25 years has the negative impact of ADT drugs on bone health in men with PCa been documented. Average declines in bone mineral density (BMD) from 2% to 10% per year have been reported in studies of men treated with LHRHa ADT medications, according to R. Langley et al. (2016).
As part of a Phase-II “PATCH” trial in the United Kingdom, Langley et al. studied the effect of using high-dose tE2 ADT on osteoporosis in men with advanced or metastatic prostate cancer (using 3-4 FEM7 patches with a nominal strength = 100 mg E2/24 hrs, changed twice weekly). In the PATCH phase-II trial, a total of 79 men on ADT were randomized to use either standard LHRHa ADT medications (e.g., Lupron), or tE2 patches for ADT. BMD scans using Dual-Energy X-Ray (DEXA) were performed at baseline, 1 year, and 2 years of continuous ADT. The median serum E2 concentration measured in the PATCH -II trial was 230 pg/ml (5%-95% range = 100 – 520 pg/ml). The lowest serum E2 measured was 70 pg/ml, and the highest serum E2 measured was 1800 pg/ml in the PATCH trials, indicating much variance.
While different sites in the body underwent different % changes in BMD over time, the greatest % changes occurred at the lumbar spine (LS). The graph below illustrates the mean % change in BMD for the LS as a function of time (months) over a 2-year period. The graph compares standard LHRHa ADT to tE2 ADT. After 2 years of ADT, the mean % change in BMD at the LS was a loss of -3.0% for the LHRHa ADT group, versus a gain of +7.9 % for the tE2 ADT group of men (p < 0.001), for a total net difference of 9.3% between the LHRHa and tE2 groups.
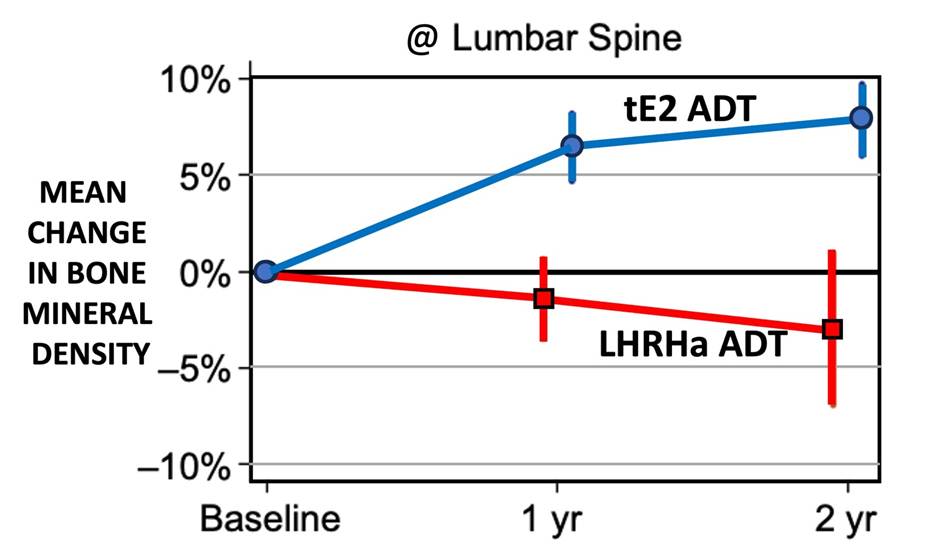
These data clearly show that high-dose tE2 ADT is protective of BMD in androgen deprived men. Unfortunately, we don’t have similar data on treating osteoporosis in men using low-to-medium doses of tE2. Based on the Sandoz data reported above for post-menopausal women, we expect the effect of tE2 on treating osteoporosis in men to be similarly dose-dependent, meaning that smaller tE2 doses will likely produce a smaller change in BMD than the high-dose tE2 results reported above from the PATCH-II trial.
Clearly, more data are needed on the effectiveness of low-to-medium dose tE2 for treating osteoporosis in men on ADT.
References:
Sandoz (Novartis). (2023). Climara™ brand estradiol patch: Patient data sheet. https://www.sandoz.com
Langley, R. E., Kynaston, H. G., Alhasso, A. A., Duong, T., Paez, E. M., Jovic, G., Scrase, C. D., Robertson, A., Cafferty, F., Welland, A., Carpenter, R., Honeyfield, L., Abel, R. L., Stone, M., Parmar, M. K., & Abel, P. D. (2016). A randomised comparison evaluating changes in bone mineral density in advanced prostate cancer: Luteinising hormone-releasing hormone agonists versus transdermal oestradiol. European Urology, 69(6), 1016–1025. https://doi.org/10.1016/j.eururo.2015.11.030
Russell, N., Ghasem-Zadeh, A., Hoermann, R., Cheung, A. S., Zajac, J. D., Shore-Lorenti, C., Ebeling, P. R., Handelsman, D. J., & Grossmann, M. (2022). Effects of estradiol on bone in men undergoing androgen deprivation therapy: A randomized placebo-controlled trial. European Journal of Endocrinology, 187(2), 241–256. https://doi.org/10.1530/EJE-22-0227
Park, J. Y., Hong, A. R., Yoon, J. H., Kim, H. K., Kang, H. C., Jung, S. I., Kwan, D., & Hwang, E. C. (2025). Alteration of bone architecture following androgen deprivation therapy in patients with prostate cancer using 3D-modeling from hip DXA. Journal of Bone Oncology, 55, 100713. https://doi.org/10.1016/j.jbo.2025.100713
